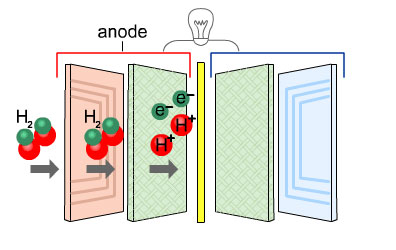
At the anode the hydrogen gas separates into protons (H+) and electrons (e-).
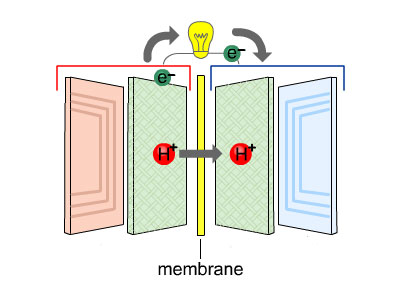
The electrons create an electric current which flows round outside the fuel cell and is used to power motors or lights.
At the same time, the protons travel through the membrane.
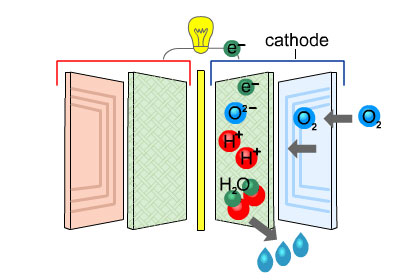
At the cathode, each oxygen molecule splits into two oxygen atoms which combine with the electrons and protons to create water and heat.
These are the only waste products.