You can think of a fuel cell as a battery that will never go flat. Electricity is made in the fuel cell by a chemical reaction just like in an ordinary battery. However in a fuel cell the reaction is between two gases, hydrogen (H2) and oxygen (O2), so the cell can be constantly re-fueled.
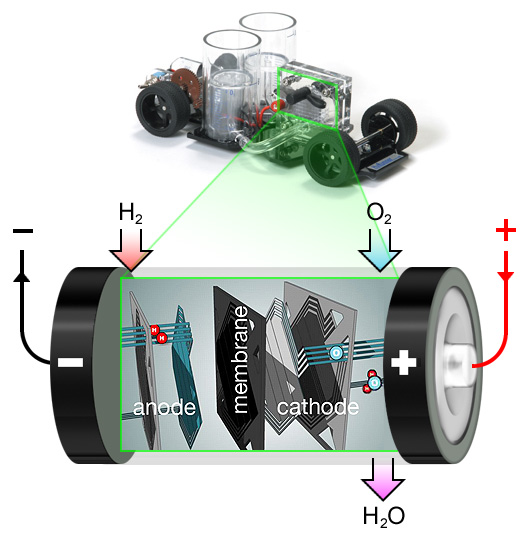
The hydrogen is stored in cars either in liquefied form or as a compressed gas. In a space-ship the oxygen would have to be stored too, but for cars we can use the oxygen in the air.
Conventional engines burn their fuel to create power directly and give off harmful green-house gases. The reaction in the fuel cell creates only electricity, which is used to power motors, and water (H2O) which is drained off.