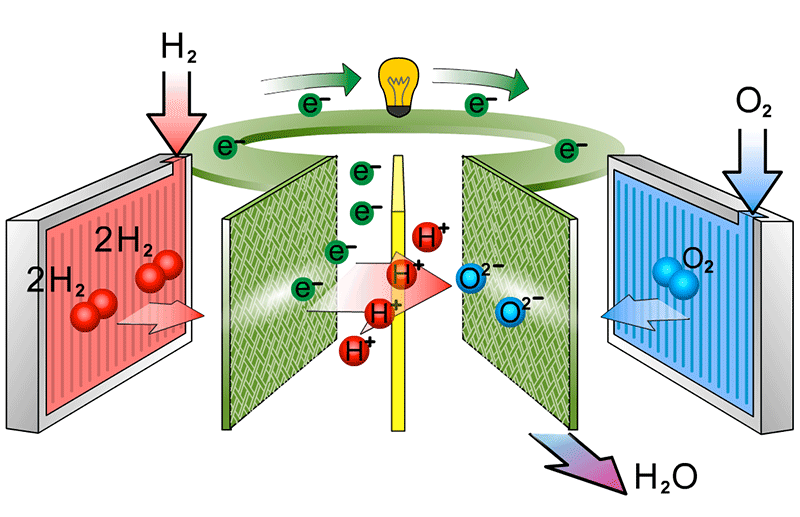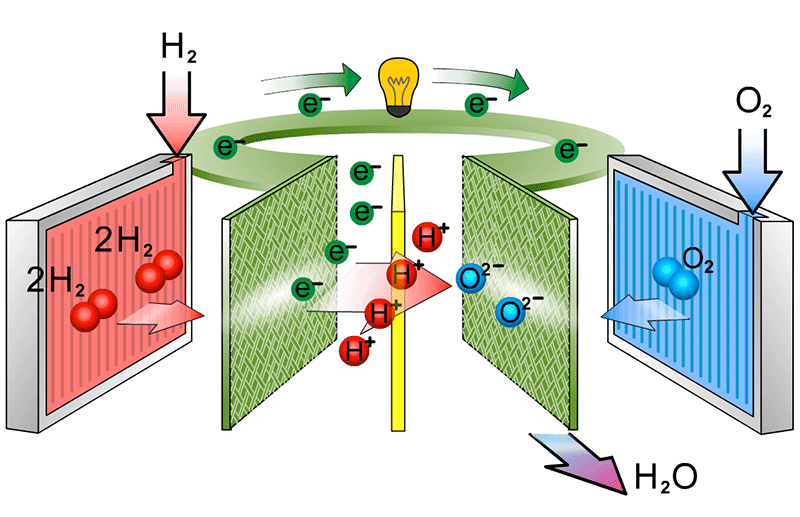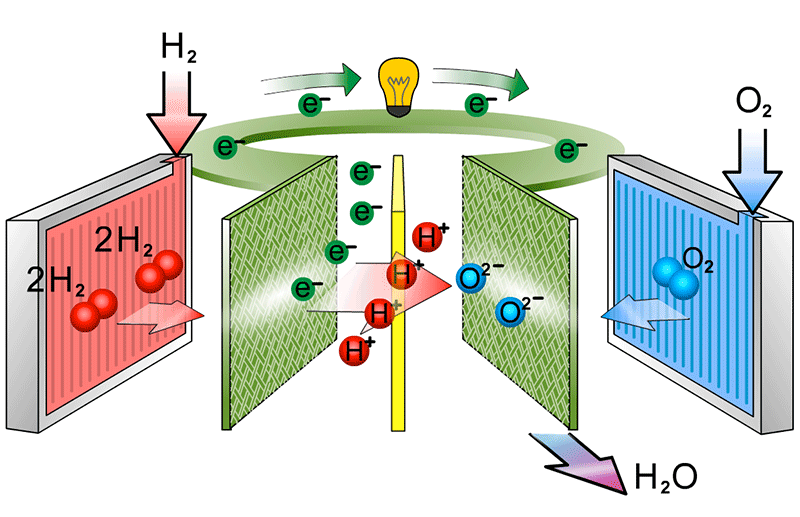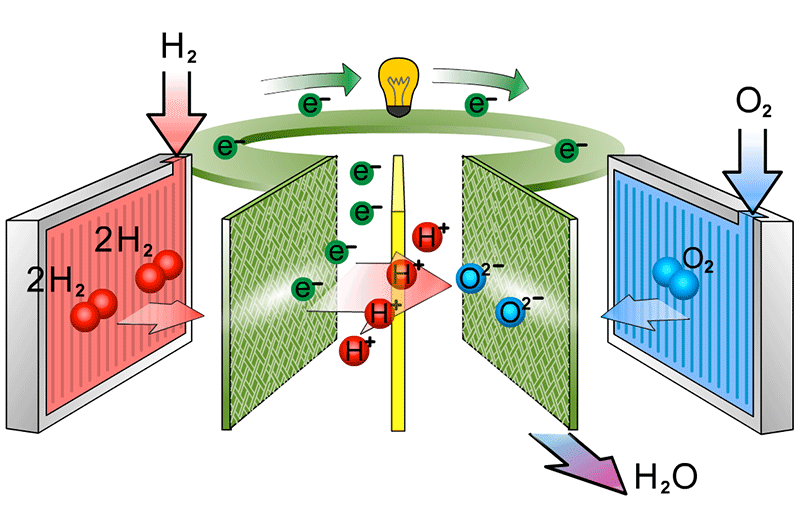
to show the catalyst equations
At the cathode, the oxygen molecules first split into two negatively charged oxygen atoms on the catalyst. These atoms then combine with electrons from the external circuit and protons that have passed through the membrane. This reaction creates water and heat, the only waste products of the fuel cell.