The membrane is a key part of the fuel cell, and is designed to only allow protons (hydrogen ions, H+) to pass through. If the electrons could pass through or if the membrane allowed the hydrogen and the oxygen to simply mix then there would be a chemical reaction creating heat and water, but no useful electric current would be created.

 In this simplified model, water molecules pass through the saturated membrane by moving from one water region to another. The excess protons on the anode side of the membrane 'hitch a ride' by forming charged hydronium ions (H3O+) and then 'jump off' at the cathode side leaving uncharged water molecules.
In this simplified model, water molecules pass through the saturated membrane by moving from one water region to another. The excess protons on the anode side of the membrane 'hitch a ride' by forming charged hydronium ions (H3O+) and then 'jump off' at the cathode side leaving uncharged water molecules.
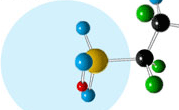 The membrane in many fuel cells is made from a polymer called Nafion which is full of water regions and has a chemical structure that encourages the transfer of protons.
The membrane in many fuel cells is made from a polymer called Nafion which is full of water regions and has a chemical structure that encourages the transfer of protons.
However other materials are being investigated that can work at higher temperatures and may provide greater efficiency.
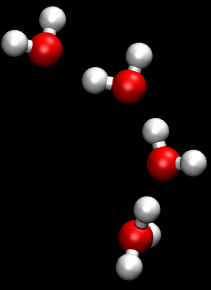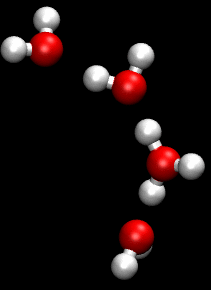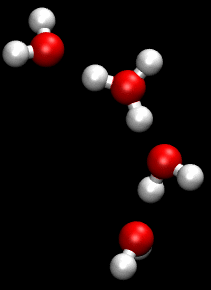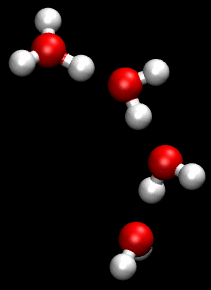 The hydronium transport model is a greatly simplified view of proton movement. More complex mechanisms involving 'proton-hopping', where the breaking and remaking of hydrogen bonds causes a rapid diffusion of protons, are also thought to be involved
The hydronium transport model is a greatly simplified view of proton movement. More complex mechanisms involving 'proton-hopping', where the breaking and remaking of hydrogen bonds causes a rapid diffusion of protons, are also thought to be involved